Chapter 31 Muraro human pancreas (CEL-seq)
31.1 Introduction
This performs an analysis of the Muraro et al. (2016) CEL-seq dataset, consisting of human pancreas cells from various donors.
31.2 Data loading
Converting back to Ensembl identifiers.
library(AnnotationHub)
edb <- AnnotationHub()[["AH73881"]]
gene.symb <- sub("__chr.*$", "", rownames(sce.muraro))
gene.ids <- mapIds(edb, keys=gene.symb,
keytype="SYMBOL", column="GENEID")
# Removing duplicated genes or genes without Ensembl IDs.
keep <- !is.na(gene.ids) & !duplicated(gene.ids)
sce.muraro <- sce.muraro[keep,]
rownames(sce.muraro) <- gene.ids[keep]31.3 Quality control
This dataset lacks mitochondrial genes so we will do without. For the one batch that seems to have a high proportion of low-quality cells, we compute an appropriate filter threshold using a shared median and MAD from the other batches (Figure 31.1).
library(scater)
stats <- perCellQCMetrics(sce.muraro)
qc <- quickPerCellQC(stats, percent_subsets="altexps_ERCC_percent",
batch=sce.muraro$donor, subset=sce.muraro$donor!="D28")
sce.muraro <- sce.muraro[,!qc$discard]colData(unfiltered) <- cbind(colData(unfiltered), stats)
unfiltered$discard <- qc$discard
gridExtra::grid.arrange(
plotColData(unfiltered, x="donor", y="sum", colour_by="discard") +
scale_y_log10() + ggtitle("Total count"),
plotColData(unfiltered, x="donor", y="detected", colour_by="discard") +
scale_y_log10() + ggtitle("Detected features"),
plotColData(unfiltered, x="donor", y="altexps_ERCC_percent",
colour_by="discard") + ggtitle("ERCC percent"),
ncol=2
)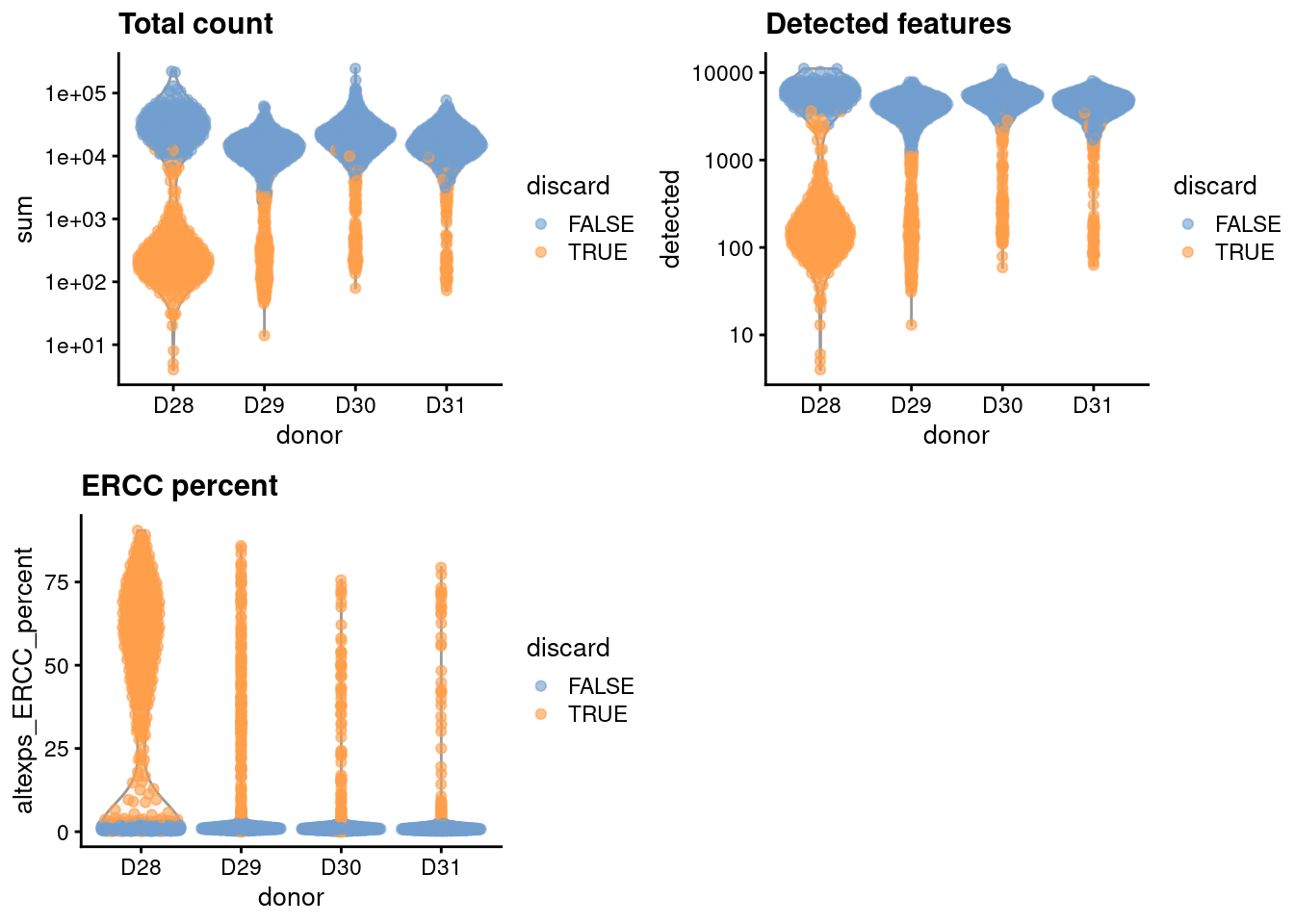
Figure 31.1: Distribution of each QC metric across cells from each donor in the Muraro pancreas dataset. Each point represents a cell and is colored according to whether that cell was discarded.
We have a look at the causes of removal:
## low_lib_size low_n_features high_altexps_ERCC_percent
## 663 700 738
## discard
## 77331.4 Normalization
library(scran)
set.seed(1000)
clusters <- quickCluster(sce.muraro)
sce.muraro <- computeSumFactors(sce.muraro, clusters=clusters)
sce.muraro <- logNormCounts(sce.muraro)## Min. 1st Qu. Median Mean 3rd Qu. Max.
## 0.088 0.541 0.821 1.000 1.211 13.987plot(librarySizeFactors(sce.muraro), sizeFactors(sce.muraro), pch=16,
xlab="Library size factors", ylab="Deconvolution factors", log="xy")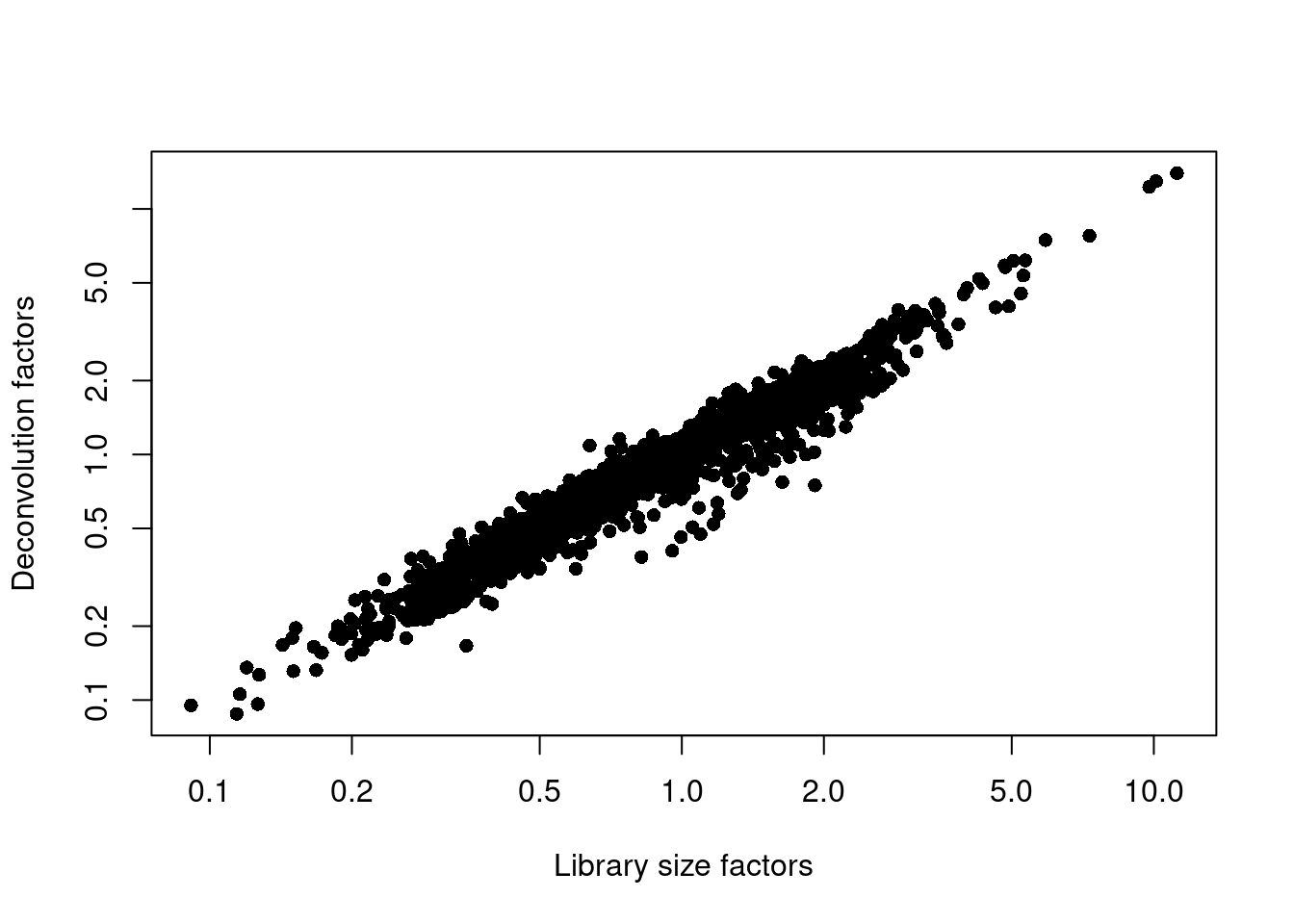
Figure 31.2: Relationship between the library size factors and the deconvolution size factors in the Muraro pancreas dataset.
31.5 Variance modelling
We block on a combined plate and donor factor.
block <- paste0(sce.muraro$plate, "_", sce.muraro$donor)
dec.muraro <- modelGeneVarWithSpikes(sce.muraro, "ERCC", block=block)
top.muraro <- getTopHVGs(dec.muraro, prop=0.1)par(mfrow=c(8,4))
blocked.stats <- dec.muraro$per.block
for (i in colnames(blocked.stats)) {
current <- blocked.stats[[i]]
plot(current$mean, current$total, main=i, pch=16, cex=0.5,
xlab="Mean of log-expression", ylab="Variance of log-expression")
curfit <- metadata(current)
points(curfit$mean, curfit$var, col="red", pch=16)
curve(curfit$trend(x), col='dodgerblue', add=TRUE, lwd=2)
}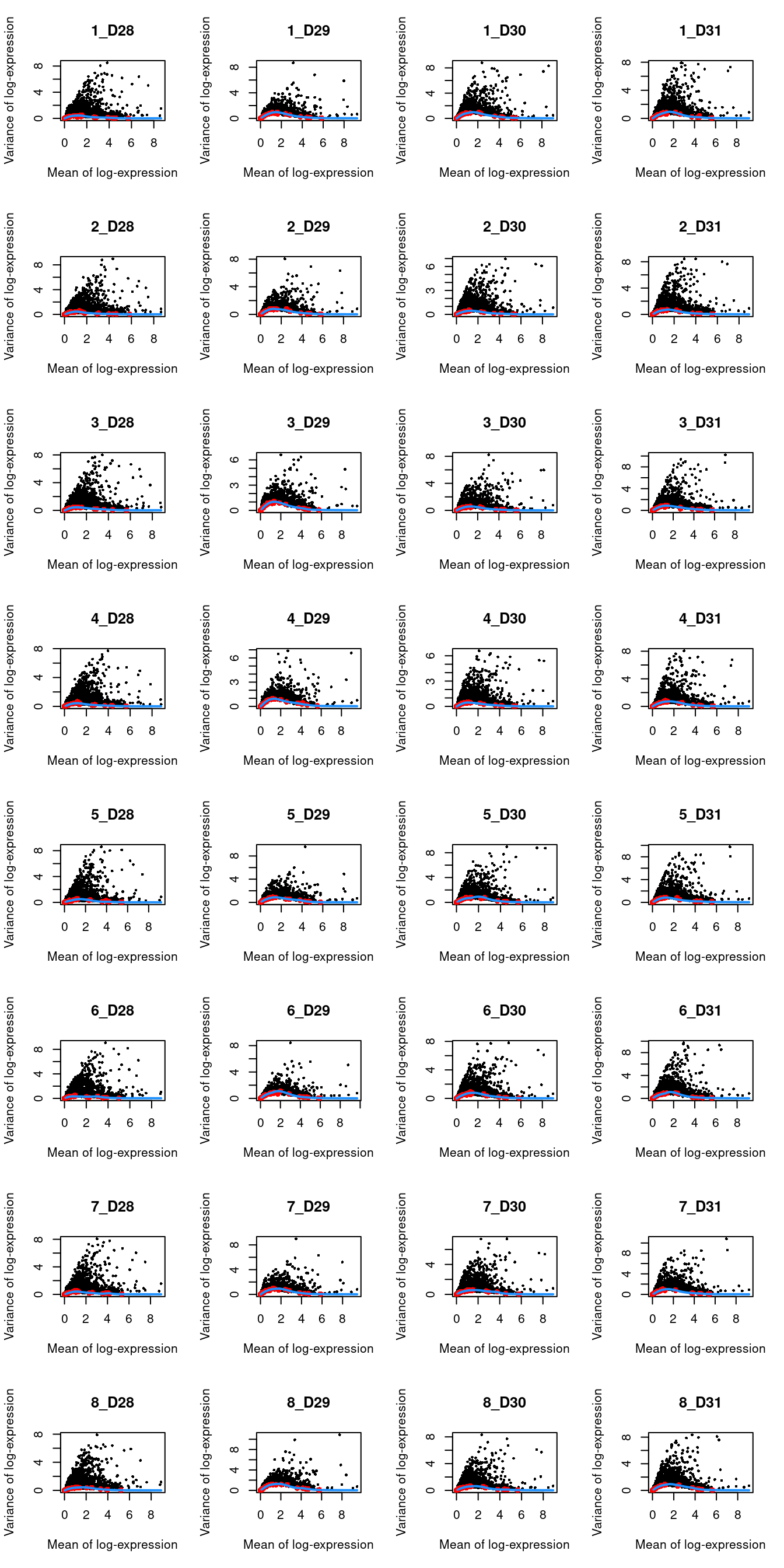
Figure 31.3: Per-gene variance as a function of the mean for the log-expression values in the Muraro pancreas dataset. Each point represents a gene (black) with the mean-variance trend (blue) fitted to the spike-in transcripts (red) separately for each donor.
31.6 Data integration
library(batchelor)
set.seed(1001010)
merged.muraro <- fastMNN(sce.muraro, subset.row=top.muraro,
batch=sce.muraro$donor)We use the proportion of variance lost as a diagnostic measure:
## D28 D29 D30 D31
## [1,] 0.060847 0.024121 0.000000 0.00000
## [2,] 0.002646 0.003018 0.062421 0.00000
## [3,] 0.003449 0.002641 0.002598 0.0816231.7 Dimensionality reduction
31.8 Clustering
snn.gr <- buildSNNGraph(merged.muraro, use.dimred="corrected")
colLabels(merged.muraro) <- factor(igraph::cluster_walktrap(snn.gr)$membership)tab <- table(Cluster=colLabels(merged.muraro), CellType=sce.muraro$label)
library(pheatmap)
pheatmap(log10(tab+10), color=viridis::viridis(100))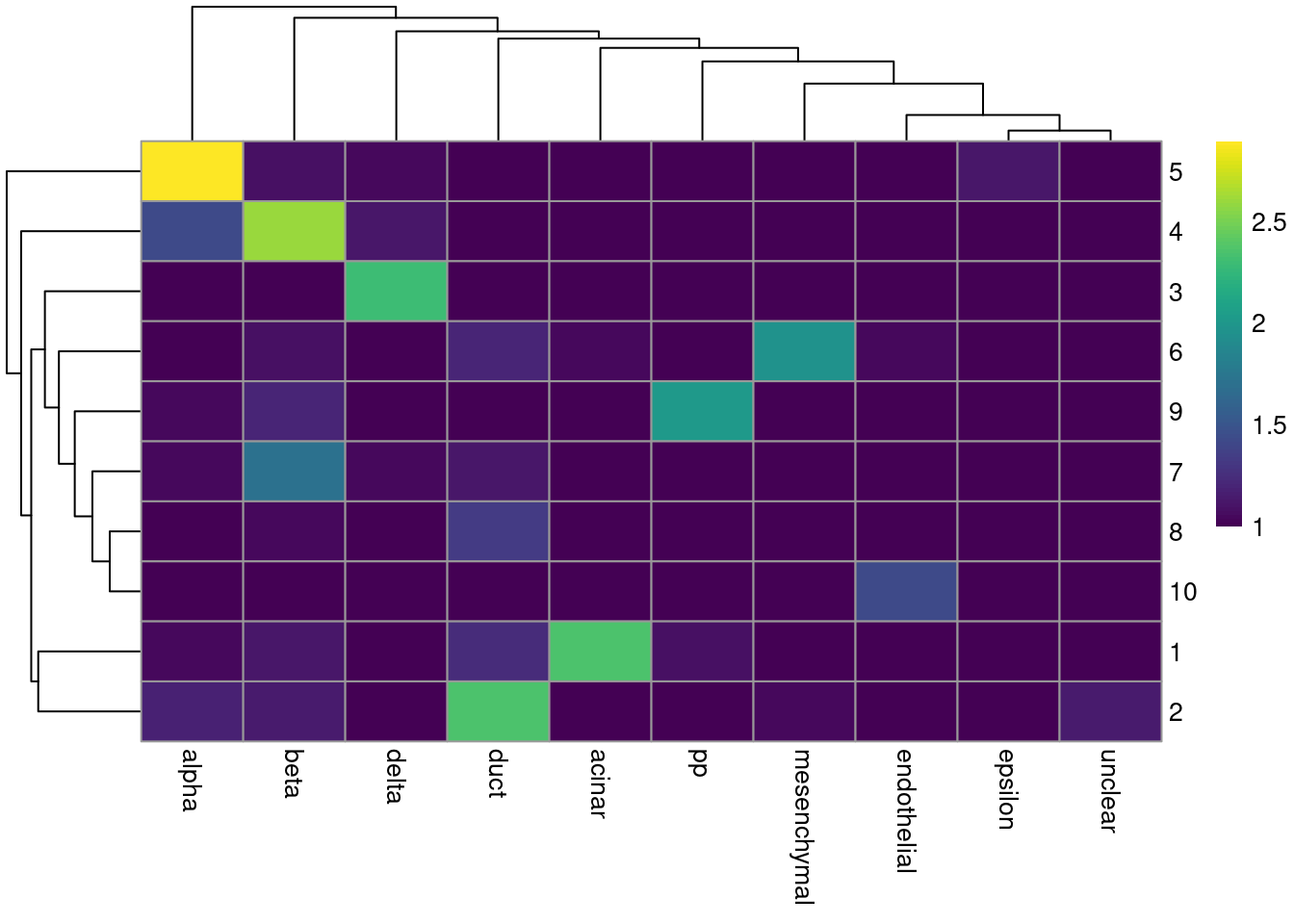
Figure 31.4: Heatmap of the frequency of cells from each cell type label in each cluster.
## Donor
## Cluster D28 D29 D30 D31
## 1 104 6 57 112
## 2 59 21 77 97
## 3 12 75 64 43
## 4 28 149 126 120
## 5 87 261 277 214
## 6 21 7 54 26
## 7 1 6 6 37
## 8 6 6 5 2
## 9 11 68 5 30
## 10 4 2 5 8gridExtra::grid.arrange(
plotTSNE(merged.muraro, colour_by="label"),
plotTSNE(merged.muraro, colour_by="batch"),
ncol=2
)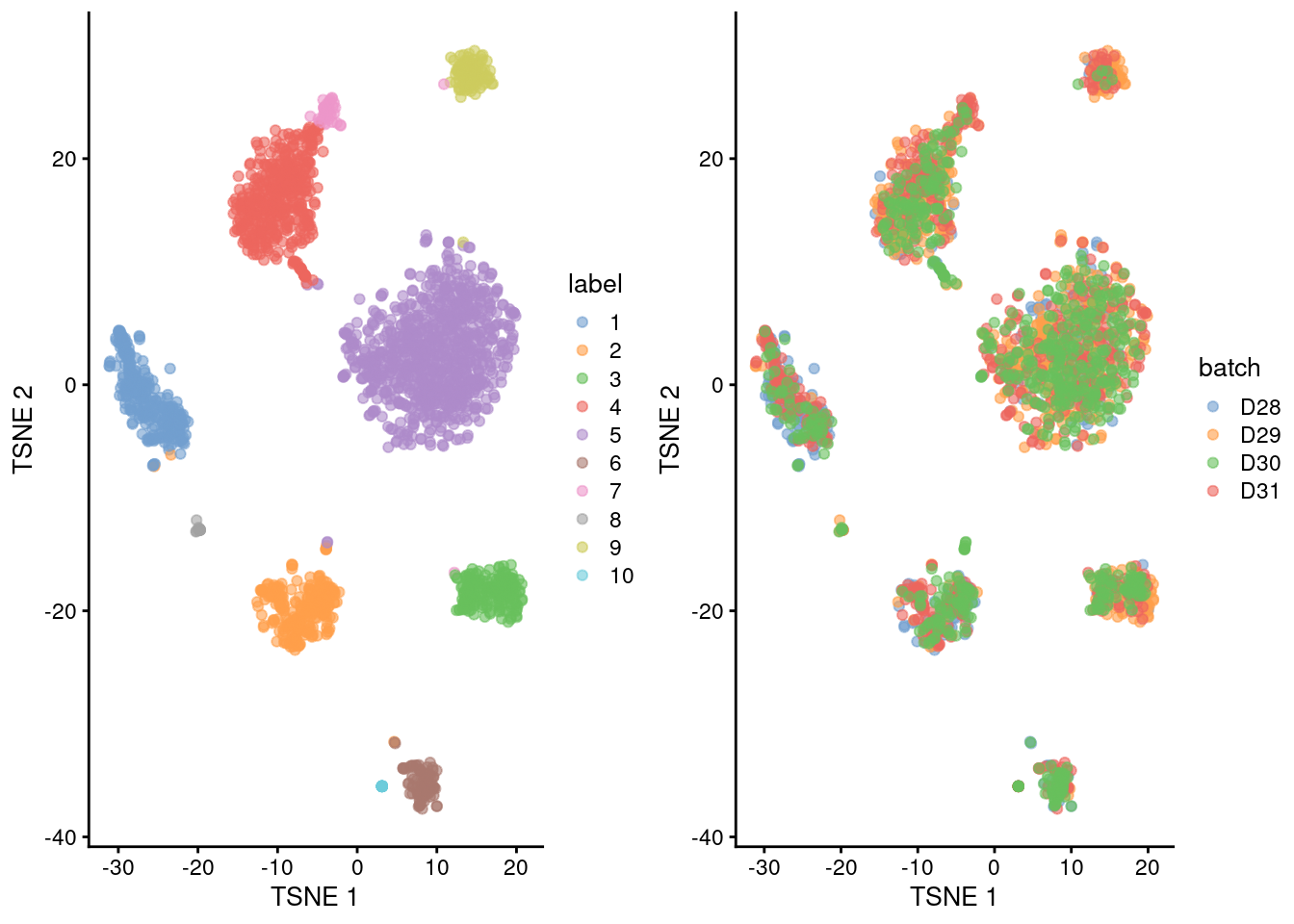
Figure 31.5: Obligatory \(t\)-SNE plots of the Muraro pancreas dataset. Each point represents a cell that is colored by cluster (left) or batch (right).
Session Info
R version 4.0.4 (2021-02-15)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Ubuntu 20.04.2 LTS
Matrix products: default
BLAS: /home/biocbuild/bbs-3.12-books/R/lib/libRblas.so
LAPACK: /home/biocbuild/bbs-3.12-books/R/lib/libRlapack.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=C
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] parallel stats4 stats graphics grDevices utils datasets
[8] methods base
other attached packages:
[1] pheatmap_1.0.12 batchelor_1.6.2
[3] scran_1.18.5 scater_1.18.6
[5] ggplot2_3.3.3 ensembldb_2.14.0
[7] AnnotationFilter_1.14.0 GenomicFeatures_1.42.2
[9] AnnotationDbi_1.52.0 AnnotationHub_2.22.0
[11] BiocFileCache_1.14.0 dbplyr_2.1.0
[13] scRNAseq_2.4.0 SingleCellExperiment_1.12.0
[15] SummarizedExperiment_1.20.0 Biobase_2.50.0
[17] GenomicRanges_1.42.0 GenomeInfoDb_1.26.4
[19] IRanges_2.24.1 S4Vectors_0.28.1
[21] BiocGenerics_0.36.0 MatrixGenerics_1.2.1
[23] matrixStats_0.58.0 BiocStyle_2.18.1
[25] rebook_1.0.0
loaded via a namespace (and not attached):
[1] igraph_1.2.6 lazyeval_0.2.2
[3] BiocParallel_1.24.1 digest_0.6.27
[5] htmltools_0.5.1.1 viridis_0.5.1
[7] fansi_0.4.2 magrittr_2.0.1
[9] memoise_2.0.0 limma_3.46.0
[11] Biostrings_2.58.0 askpass_1.1
[13] prettyunits_1.1.1 colorspace_2.0-0
[15] blob_1.2.1 rappdirs_0.3.3
[17] xfun_0.22 dplyr_1.0.5
[19] callr_3.5.1 crayon_1.4.1
[21] RCurl_1.98-1.3 jsonlite_1.7.2
[23] graph_1.68.0 glue_1.4.2
[25] gtable_0.3.0 zlibbioc_1.36.0
[27] XVector_0.30.0 DelayedArray_0.16.2
[29] BiocSingular_1.6.0 scales_1.1.1
[31] edgeR_3.32.1 DBI_1.1.1
[33] Rcpp_1.0.6 viridisLite_0.3.0
[35] xtable_1.8-4 progress_1.2.2
[37] dqrng_0.2.1 bit_4.0.4
[39] rsvd_1.0.3 ResidualMatrix_1.0.0
[41] httr_1.4.2 RColorBrewer_1.1-2
[43] ellipsis_0.3.1 pkgconfig_2.0.3
[45] XML_3.99-0.6 farver_2.1.0
[47] scuttle_1.0.4 CodeDepends_0.6.5
[49] sass_0.3.1 locfit_1.5-9.4
[51] utf8_1.2.1 tidyselect_1.1.0
[53] labeling_0.4.2 rlang_0.4.10
[55] later_1.1.0.1 munsell_0.5.0
[57] BiocVersion_3.12.0 tools_4.0.4
[59] cachem_1.0.4 generics_0.1.0
[61] RSQLite_2.2.4 ExperimentHub_1.16.0
[63] evaluate_0.14 stringr_1.4.0
[65] fastmap_1.1.0 yaml_2.2.1
[67] processx_3.4.5 knitr_1.31
[69] bit64_4.0.5 purrr_0.3.4
[71] sparseMatrixStats_1.2.1 mime_0.10
[73] xml2_1.3.2 biomaRt_2.46.3
[75] compiler_4.0.4 beeswarm_0.3.1
[77] curl_4.3 interactiveDisplayBase_1.28.0
[79] statmod_1.4.35 tibble_3.1.0
[81] bslib_0.2.4 stringi_1.5.3
[83] highr_0.8 ps_1.6.0
[85] lattice_0.20-41 bluster_1.0.0
[87] ProtGenerics_1.22.0 Matrix_1.3-2
[89] vctrs_0.3.6 pillar_1.5.1
[91] lifecycle_1.0.0 BiocManager_1.30.10
[93] jquerylib_0.1.3 BiocNeighbors_1.8.2
[95] cowplot_1.1.1 bitops_1.0-6
[97] irlba_2.3.3 httpuv_1.5.5
[99] rtracklayer_1.50.0 R6_2.5.0
[101] bookdown_0.21 promises_1.2.0.1
[103] gridExtra_2.3 vipor_0.4.5
[105] codetools_0.2-18 assertthat_0.2.1
[107] openssl_1.4.3 withr_2.4.1
[109] GenomicAlignments_1.26.0 Rsamtools_2.6.0
[111] GenomeInfoDbData_1.2.4 hms_1.0.0
[113] grid_4.0.4 beachmat_2.6.4
[115] rmarkdown_2.7 DelayedMatrixStats_1.12.3
[117] Rtsne_0.15 shiny_1.6.0
[119] ggbeeswarm_0.6.0 Bibliography
Muraro, M. J., G. Dharmadhikari, D. Grun, N. Groen, T. Dielen, E. Jansen, L. van Gurp, et al. 2016. “A Single-Cell Transcriptome Atlas of the Human Pancreas.” Cell Syst 3 (4): 385–94.